Prof.Wu’s research group published an invited review in ChemBioChem
On 27th of August 2018, Dr. Fang Wu’s group published an invited review article entitled “The Multiplicity of Polypeptide GalNAc-Transferase: Assays, Inhibitors and Structures”, which was kindly requested from Dr. Ragg, the executive chief editor of ChemBioChem.
The review systematically summarizes the research progresses of Polypeptide GalNAc-Transferase (GalNAc-T) on the catalytic mechanism, biochemical assays, small-molecule inhibitor and 3D crystal structures aspects during the last few decades. Base on the analysis of the current knowledge and the superimposed crystal structures of four isoform GalNAc-Ts, the authors originally propose a novel molecular mechanism underlying the activity-regulation of GalNAc-Ts, which provide new clues for elucidating the key remaining questions for GalNAc-T, e.g. the catalytic mechanism of GalNAc-Ts, the recognition mechanisms and selectivities between GalNAc-Ts and their substrates or inhibitors. The 43-pages review, including 9 figures or tables and 106 cited references, was completed by reading hundreds of publications in the related fields.
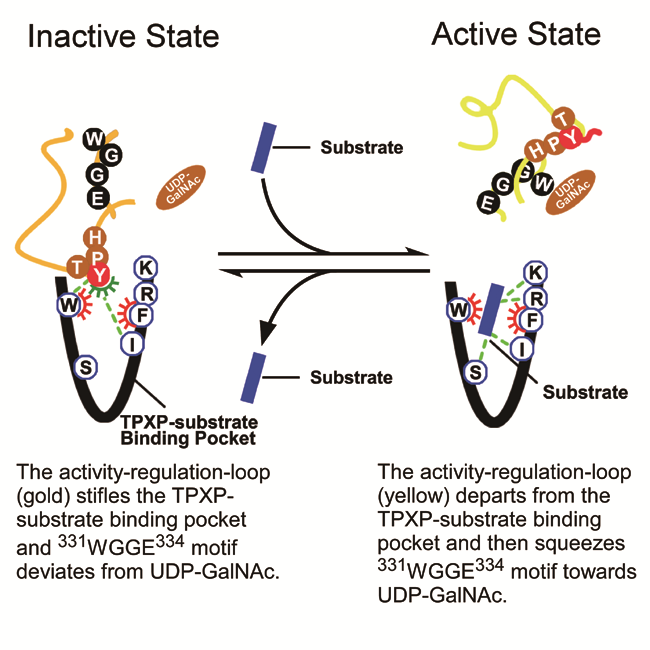
In the review, the authors propose that the activity-regulation-loop and TPXP-substrate binding pocket of GalNAc-Ts play important roles in the activity regulation and substrates- and inhibitors- recognition processes. The activity-regulation-loop as well as substrates and small-molecule inhibitors can interact with the common residues in TPXP-substrate binding pocket and therefore determine the function and activation status of enzymes. When the activity-regulation-loop stifles the binding pocket, the catalytic WGGE motif deviates from the donor substrate UDP-GalNAc, which keeps the enzyme in an inactive status. Once the acceptor substrates or substrate- competitive inhibitors seize the pocket, the activity-regulation-loop would depart from the binding pocket, and the alpha-helical terminus of the activity-regulation-loop will squeeze the catalytic WGGE motif towards the donor substrate UDP-GalNAc and therefore activates GalNAc-Ts. The affinities of the substrates and inhibitors towards the binding pocket determine their selectivities to different isoenzymes. The new proposed mechanism will likely generate important breakthroughs in developing novel drug leads targeting GalNAc-Ts.
The review was independently accomplished by Dr. Fang Wu’s group. Dr. Youtian Hu is the first author, Dr. Fang Wu is the corresponding author, and PhD candidate Juan Feng also contributes to the review. This review was published online ahead of print in ChemBioChem (https://www.ncbi.nlm.nih.gov/pubmed/30152088) on 27th August, 2018. This work was supported by the National Basic Research Program of China (No. 2012CB822103) and the National Natural Science Foundation (Grant Number 31500635). Dr. Fang Wu’s group is now employing this novel mechanism to investigate the structure-function relationship and develop specific inhibitors as well as new substrates for GalNAc-Ts. The review was also highlighted by X-MOL.