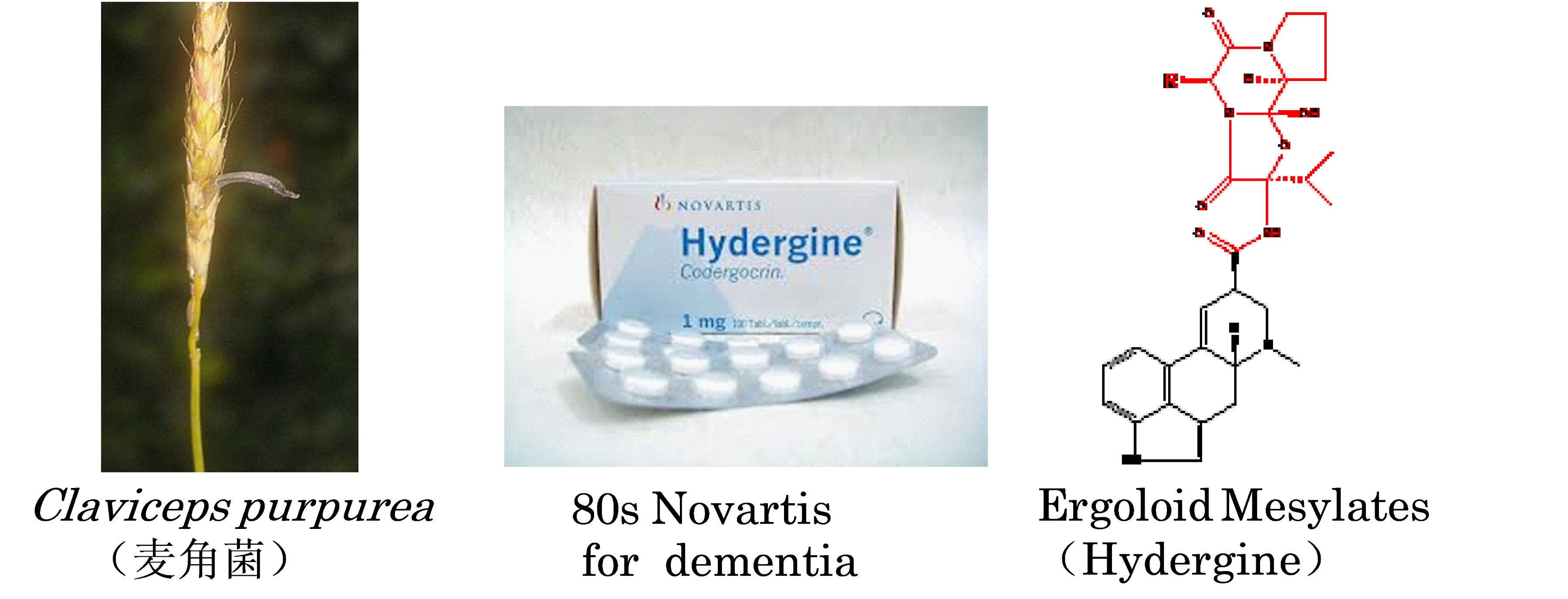
FDA-approved natural product hydergine for treatment of Alzheimer's disease
With the increase of the average human life span, the incidence of age-related disease is increasing year by year. Alzheimer's Disease (AD) in older age people is the most common neurodegenerative disease. Now, there is about 44 million Alzheimer's patients worldwide. But unfortunately, there is still no effective treatment for AD. According to statistics for recent years, the failure rate for AD drug development is as high as 99.6%.
Our group at Shanghai Center for Systems Biomedicine screened 417 natural products, and discovered a natural product from the ergot fungus (Claviceps purpurea), i.e dihydroergocristine, could reduce the production of Abeta peptide in cells from an AD patient. Interestingly, the natural product is an active component of the FDA-approved drug Hydergine ®. Hydergine was approved for treatment of age-related dementia and marketed as Hydergine ® in Switzerland by Novartis company. The mechanism of action of it is not clear, and it has not been widely applied in the treatment of AD. Our group used biochemical and molecular biological methods to describe the target of the older drug dihydroergocristine is via lowering abeta and offers a new trick of the old drug for repurposing of Hydergine for treatment of Alzheimer's disease. This study is published in the Scientific Reports, a multidisciplinary open journal, and the new indication of the natural product has also been patented. The work is carried out by Master student Lei Xiling and Teacher Yu Jing in the group (www.nature.com/articles/srep16541).