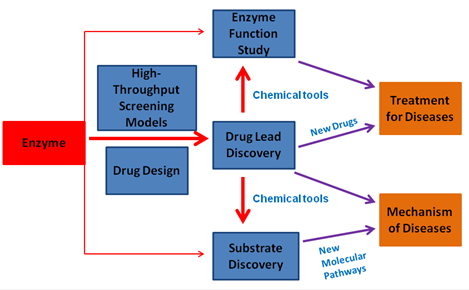
Small-molecule drugs and Enzyme target
There are ~1,500 small molecule drugs currently marketed, accounting for ~ 80% of total FDA-approved drugs. Small-molecule drugs target 317 kinds of enzymes, which is ~ 22 % of the total drug targets. Thus, developing of small-molecule inhibitors or activator for enzymes is essential for treatment of diseases.
Our laboratory use biochemistry, high throughput screening and drug design as well as other research tools to discover small molecule modulators of enzymes, aiming for developing drug leads and chemical tools for the relevant diseases. These agents can be used as a drug lead for the treatment of disease, and chemical probes for clarifying the underlying mechanism of diseases.
Currently, our lab has identified several selective and bioactive small-molecule inhibitors for a variety of enzymes involved in neurodegenerative disease, drug-resistance of bacteria, immunlogical disorders and cancer. For example, the first specific and bioactive inhibitor of CBS (a vitamin B6 -dependent enzyme) has been discovered for combating cancer (Wang l et al, Cell Death and Disease 2018; Zhou Y. et al, Chem Comm 2013). The inhibitor represents the most potent inhibitor of CBS to date, and could significantly inhibits the growth of tumor cells in a liver cancer xenograft model. By employing this pharmacological probe, we could uncover that CBS is a direct negative regulator for ferropotosis in liver cancer cells; For an emerging mucin-type O-glycosylation target, GalNAc-T enzyme, we identified a new class of bioactive inhibitors(Luteolin、Quercetin和Myricetin)by HTS screening of natural products. The inhibitor Luteolin could competitively with the peptide substrate and is the first substrate-competitive inhibitor of GalNAc-T (Liu F. and Xu K. et al, J. Bio. Chem 2017). The inhibitor could substantially reduce the production of Abeta in a APP/PS model of Alzheimer’s disease. After studying and analyzing the bind mode of Luteolin and the available crystal structures of GalNAc-T, we could reveal a new mechanism of activity regulation for GalNAc-T (ChemBioChem, 2018; Invited Review); We also identified a natural-product-based inhibitor for Parkinson 's disease drug target DDC (B6 enzyme; Ren J. et al, ACS Chem Biol, 2014, Cover Story); By using the drug repositioning strategy, we could found that the old drug HydergineÒ has the ability to reduce the Abea production in cells derived from Alzheimer’s disease patients (Lei XL. et al, Sci Rep 2015).
High-throughput drug screening model
High-throughput drug screening in the pharmaceutical industry is currently widely used, and is the commonly used method to find drug leads. For example, the drug star Gleevec was screened out by this approach. Constructing innovative drug screening model is the essential for high-throughput drug screening. We constructed several targeted-based high-throughput screening models for a few diseases, including cancer, Alzheimer's disease, Parkinson's diseases, autoimmune diseases, hypertension and other diseases. Apart from the innovative models, we also collected 40,000 small molecules, including 4,000 special natural products from Chinese plants or animals.
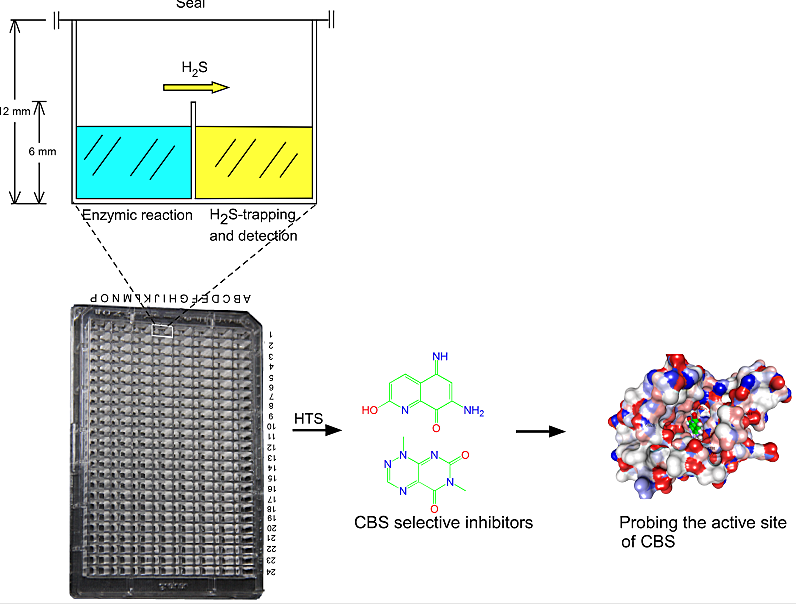
Figure 1. The illustration for the tandem-well-based screening of inhibitors for CBS.
The enzymes that produce gaseous signaling molecules e.g. NO, CO and H2S, is considered to be potential drug targets. In the past, high-throughput method for the detection of gas is absence, thus no specific inhibitors or activators have been found. To monitor the activity of hCBS or hCSE, the H2S gas generating enzymes, in a high-throughput mode, we designed a tandem-microwell-based assay for measuring H2S production. The newly designed 192-tandem-well plate was remodeled from a conventional 384-well assay plate by interconnecting each two adjacent wells with a channel in the upper part of the interjacent wall to allow gas exchange. In this arrangement, the gas generated in the well containing the enzymatic reaction solution is simultaneously trapped and quantified in the adjacent well (Fig. 1). The tandem microwell obviates any interference of the analytical reagent with the enzyme(s) and its (their) substrate(s). (Zhou Y. et al, Chem Comm 2013). By using this screening model, we have successfully identified the first selective inhibitor for CBS.
Moreover, we also use the tandem-enzyme-coupling strategy to develop new HTS assays for GalNAc-T and dopa decarboxylase (Liu F. and Xu K. et al, J. Bio. Chem 2017; Ren J. et al, ACS Chem. Biol., 2014). Also, several cell-based assays were successfully constructed for monitoring the cellular activity of several new enzymes, and provide assay tools for studying the function and mechanism of these enzymes..
New enzyme substrates and signaling pathway
Protein degradation in cells is a complex biological process and important issue. Transmembrane protein is also to be cut into fragments in the cell membrane and the cytoplasmic fragment sometime translocated into the nucleus, which plays a transcriptional regulatory mechanism. The whole process is called membrane regulated proteolytic cleavage (Regulated Intramembrane Proteolysis, RIP). In mammalian cells, gamma-secretase is responsible for the RIP process. APP protein is an important membrane protein for Alzheimer’s disease, and cleavaged by gamma-secretase in the membrane.
Our laboratory uses small molecular chemical tools, the molecular genetics tools, cell biology methods, to discover the new substrates for gamma-secretase and study the new function of the cellular fragment of the new substrate. It could offer new insights to the underlying mechanisms of complex disease, including Alzheimer’s disease.
Recently, we found that the homeostasis of AXL was controlled by alpha-secretase, gamma-secretase and proteasome. Moreover, the AXL receptor can be sequentially cleaved by alpha- and gamma-secretase to produce intracellular domain (ICD) that further modulate the gene expression by a newly identified nuclear localization signal of HRRKK into the nucleus. The preclinical drug, R428, can inhibit the phosphorylation of AXL, and then break the homeostasis of AXL in membrane, which accelerate the AXL cleavage by secretases, and lead to the AXL receptor down-regulation. Inhibition of AXL intramembrane proteolysis mediated by gamma-secretase significantly increases the drug resistance of Tarceva® in non-small cell lung cancer cells.
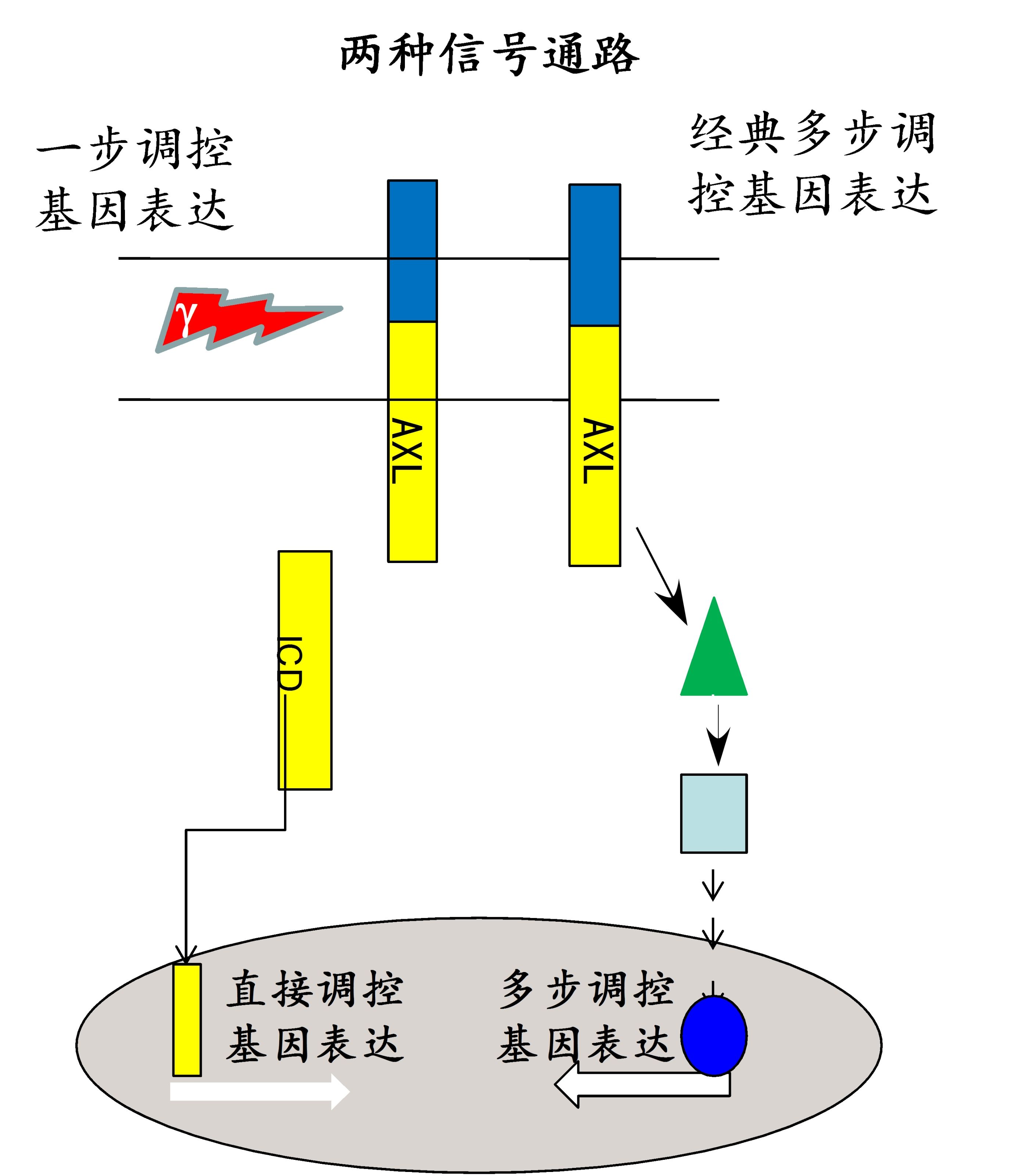
Figure 2. The canonical and non-canonical pathways of AXL signaling.
Enzyme function and chemical biology research
The catalytic mechanism of the enzymes, as well as their roles in the cellular metabolism and signal pathway, remains largely unknown. Studying of these unknown issues is important. We use small-molecular probes and chemical biology methods to tackle these problems. Using structurally diverse small molecules, combined with molecular modeling and enzyme kinetics method, to reveal enzyme substrate binding and catalytic mechanism; using high affinity/covalent labeling method to find the new target for disease and enzyme active sites; using small-molecular probes to investigate the new functions of signaling pathway and protein post-modifications.